
OPTIFLEX GENESIS COMFORT NY
Preloaded Monofocal Hydrophobic Aspheric (Natural Yellow) Foldable Intraocular Lens
OPTIFLEX GENESIS COMFORT NY is a Pre-loaded IOL system with Monofocal Hydrophobic Aspheric Natural Yellow acrylic foldable single piece posterior chamber intraocular lens.
FEATURES
Improved Visual Clarity and Contrast Sensitivity: By correcting spherical aberrations and reducing glare, Optiflex Genesis Comfort NY enhances visual clarity and contrast sensitivity, allowing patients to perceive details more accurately and comfortably in various lighting conditions
Unique Delivery System: Optiflex Genesis Comfort NY’s unique delivery system incorporates a gear mechanism that allows for precise and controlled placement of the lens, ensuring optimal positioning within the eye. The lens’s unique delivery system and high-quality materials ensure optimal visual outcomes and patient satisfaction by minimizing the risk of postoperative complications.
High Refractive Index Material: Optiflex Genesis Comfort NY material possesses a high refractive index, which contributes to make the lens optic thin enabling the implantation through smaller incision
360-Degree Square Edge: Optiflex Genesis Comfort NY features a 360-degree square edge design, which helps to minimize the risk of posterior capsular opacification (PCO) by inhibiting lens epithelial cell migration.
UV and Violet Blue Light Filtering: Optiflex Genesis Comfort NY includes filters to block harmful UV and violet-blue light, helping to protect the retina and maintain ocular health.
Significant improvement in spherical equivalent
Improved contrast sensitivity
Unique delivery system with gear mechanism for controlled IOL delivery
Material with high refractive index
360 degree square edge
Fliters UV & violet blue light
INGREDIENTS
Optiflex Genesis Comfort NY intraocular lens primarily consists of hydrophobic acrylic material augmented with natural yellow chromophore. This acrylic compound, known for its optical clarity and biocompatibility, forms the core of the lens, offering stability within the eye while minimizing the risk of posterior capsular opacification. The incorporation of a natural yellow chromophore enhances the lens’s ability to filter harmful blue light and improve contrast sensitivity, particularly in low-light conditions. Additionally, the lens includes UV and blue light filters to shield the retina from potential damage caused by ultraviolet and violet-blue light wavelengths.
OPERATIONS
Optiflex Genesis Comfort NY is specifically designed for use in cataract surgery. It is implanted into the capsular bag following the removal of the cataractous crystalline lens. The lens is suitable for patients undergoing standard cataract extraction procedures.
ADVANTAGES
Ease of Use: Optiflex Genesis Comfort NY’s pre-loaded delivery system simplifies the surgical process and reduces the risk of contamination, ensuring sterility from the factory to the operating room.
Precise Control: The first screw injector design of Optiflex Genesis Comfort NY, enables single-hand operation, providing surgeons with complete control over the lens delivery process.
Compatibility: Optiflex Genesis Comfort NY is compatible with 2.2 mm incisions, minimizing surgical trauma and promoting faster recovery times for patients.
Reduced Injection Pressure: Optiflex Genesis Comfort NY requires lower injection pressure compared to traditional delivery systems, reducing the risk of complications such as endothelial damage.
Ergonomic Design: The ergonomic design of Optiflex Genesis Comfort NY, facilitates comfortable handling during surgery, reducing surgeon fatigue and improving procedural efficiency.The delivery system of Optiflex Genesis Comfort NY enables the lens to be inserted directly into the eye without requiring additional surgical manipulations, thereby reducing the risk of postoperative complications like decentration or dislocation.
Long-Term Ocular Health and Functionality: By filtering harmful UV,violet-blue light and minimizing chromatic aberrations, Optiflex Genesis Comfort NY promotes long-term ocular health and functionality, and preserving visual acfuity over time.
TECHNICAL SPECIFICATIONS
| MODEL | LMFA6 |
| MATERIAL | Hydrophobic Acrylic containing Natural Yellow Chromophore |
| OPTIC TYPE | Single Piece, 360° Square Edge with Aspheric Optic |
| OPTIC SIZE | 6.00 mm |
| OVERALL SIZE | 13.00 mm |
| ANGULATION | 0 |
| ACD | 5.28 |
| REFRACTIVE INDEX | 1.524 |
| RECOMMENDED ULTRASOUND A-CONSTANT | SRK – T : 118.40 |
| RECOMMENDED OPTICAL A-CONSTANTS | SRK – T : 118.80 SRK – II : 119.15 HOLLADAYl 1 SF : 1.61 HOLLADAY 2 SF: 1.68 HOFFER – Q ACD : 5.39 HAIGIS – a0:1.177, a1:0.400, a2:0.100 Barrett: 1.78 |
| DIOPTER RANGE | +5.0 to +30.0 D (with 0.5 D steps) |
| CYLINDER RANGE | 0.0 D to 6.0 D (with 1.0D step between 0.0D to 1.0D, with 0.5D step between 1.0D to 1.5D & with 0.75D step between 1.5D to 6.0D) |
| IMPLANTATION SITE | Capsular Bag |
| STERILIZATION | EO |
| SHELF LIFE | 3 years from date of manufacture |
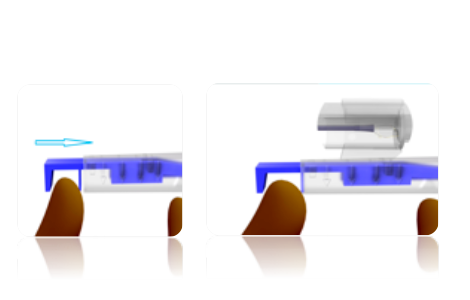
STEP 1
Push the blue injector plunger forward until the front push plate is flush against the injector housing

STEP 2
Inject adequate amount of any Biotech certified OVD having low to moderate viscosity, as shown here. The OVD should flow up to leading haptic of the IOL. Inject OVD from tip of the cartridge also, to fill the cartridge nozzle
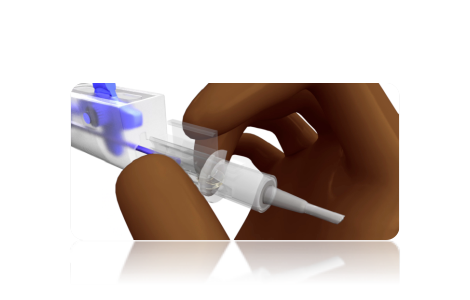
STEP 3
Close the cartridge flaps. Ensure that the flaps are locked with a “Click” sound
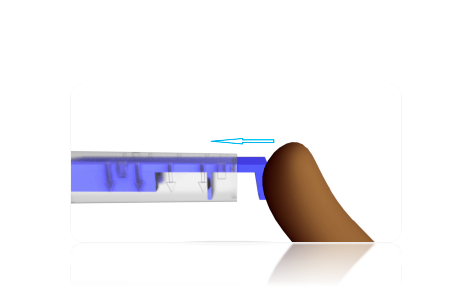
STEP 4
Push the blue injector plunger forward until the rear push plate is flush against the injector housing or until the wheel of the injector moves
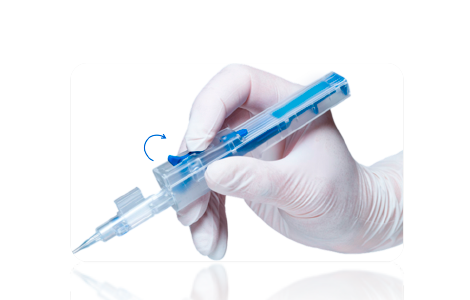
STEP 5
Hold the delivery system with a “Pen Grip”, as shown here and keep your index finger on Drive Wheel
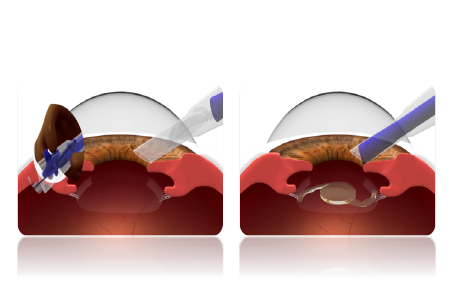
STEP 6
Insert the cartridge tip into the eye with a bevel down position & using your index finger, pull & rotate the drive wheel back slowly in order to push the lens forward until it is delivered in the eye
SURGERY VIDEO
ADDITIONAL PRODUCTS
OPTIFLEX GENESIS COMFORT is available as pre-loaded delivery system with clear hydrophobic aspheric IOL, for special orders in this segment.
Categories
Company
Media
Follow Us
© Copyright Biotech /Terms Of Use - Privacy Policy
Version 2_CT_1212222
